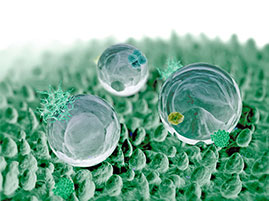
Researchers have developed a slow-release, inflammation-targeting hydrogel for patients with chronic inflammatory bowel disease (IBD), which could reduce uncomfortable, enema-based treatment from once-daily to once-weekly. Investigators from Brigham and Women’s Hospital (BWH), Massachusetts General Hospital (MGH), and Massachusetts Institute of Technology (MIT) sought to deliver anti-inflammatory treatment directly to the surface of the colon. “We realized that if we could develop a disease-targeted hydrogel system that rapidly attaches to ulcers and slowly releases drugs at the site of inflammation, then we could create a better way to deliver medicine only where the drug is needed,” said the co-corresponding author. For this study, the investigators developed hydrogel microfibers from ascorbylpalmitate, an FDA-approved compound that possesses both hydrophilic and lipophilic properties. This negatively-charged hydrogel can be disassembled by enzymes found only in inflamed tissue, which is positively charged. The investigators loaded the hydrogel with dexamethasone, which is commonly used to treat IBD. When the inflammation-targeting hydrogel drug meets such enzymes, the molecules that make up the gel begin to break apart, slowly releasing the dexamethasone.
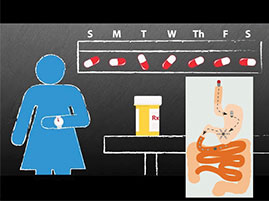
The problem with pills is that you have to take them on a regular basis. Now, imagine swallowing a pill today that continues releasing the daily dose of a medicine you need for the next week, month or even longer. Investigators from Brigham and Women's Hospital and their collaborators from the Massachusetts Institute of Technology (MIT) have developed a long-acting drug delivery capsule that may help to do just that in the future. The capsule is about the size of a fish oil capsule when swallowed. Once inside the stomach, the capsule unfolds into a star-shaped structure too large to pass through the pylorus and exit the stomach, but designed to allow food to continue passing through the digestive system. The capsule contains polymers and other materials mixed with ivermectin to allow the drug to slowly diffuse out of the material over time. The team reports evidence of diffusion for up to two weeks, and is interested in continuing to develop the system so that it can provide the drug for one month or longer. In addition, they envision potential applications beyond infectious disease, including chronic diseases such as psychiatric disease, heart disease, renal disease and more. They plan to investigate the system's applications for these conditions as well.
2016 © The Pharma World. All Rights Reserved.